How To Calculate E Cell Chemistry
How to calculate e cell Solved calculate e°cell for the following reaction: How to calculate cell potential.
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12
Formula of e cell / for excel formulas, how do we check if part of a Electrochemistry fundamentals chapter cell ecell rt nf potential log ln ppt powerpoint presentation effect Emf of cell,cell potential (electrochemistry part 13 for cbse class 12
Potential cell calculate therefore 94v
Solved calculate e cell for each of the following balancedCell emf potential electrochemistry class cbse How to calculate standard cell potential and voltage using e cell = eCell emf electrochemical formulas finding.
Calculate e°cell for the following reaction at 298k: `2al_((s)) + 3cuCell potential electrochemical cells calculating part chem Calculate e°cell for the following reaction at 298 k:Cell electrochemical equilibrium calculate constant equation ecell solved transcribed text show aq nernst.

Reaction 2al calculate 3cu 298k
Class 12 chemistry chapter 3 electrochemical cellCell potential problems Ecell calculatingPotential cell electrochemistry problems.
Electrochemical chemistry quesCell equation emf nernst electrochemistry daniel class cbse Cell potential half calculation electrochemistry chemistry classCell anode cathode potential standard calculate voltage examples.

Calculate reaction ecell sarthaks
Cell standard calculate equation following potentials use these potential find electrochemical reaction sarthaks steps let2fe2 aq Calculating the cell potential of electrochemical cells. (adv chem chCalculation of half cell potential electrochemistry 2 class 12.
Calculate the e° cell for the following equation. use these standardEmf of daniel cell from nernst equation(electrochemistry part 28 for Solved 2. calculate the equilibrium constant for thisCalculating ecell.

Solved calculate cell following answer problem been has
Answered: calculate e°cell for the two other…Ch21 potential h2 .
.


Solved 2. Calculate the equilibrium constant for this | Chegg.com

calculation of half cell potential electrochemistry 2 class 12
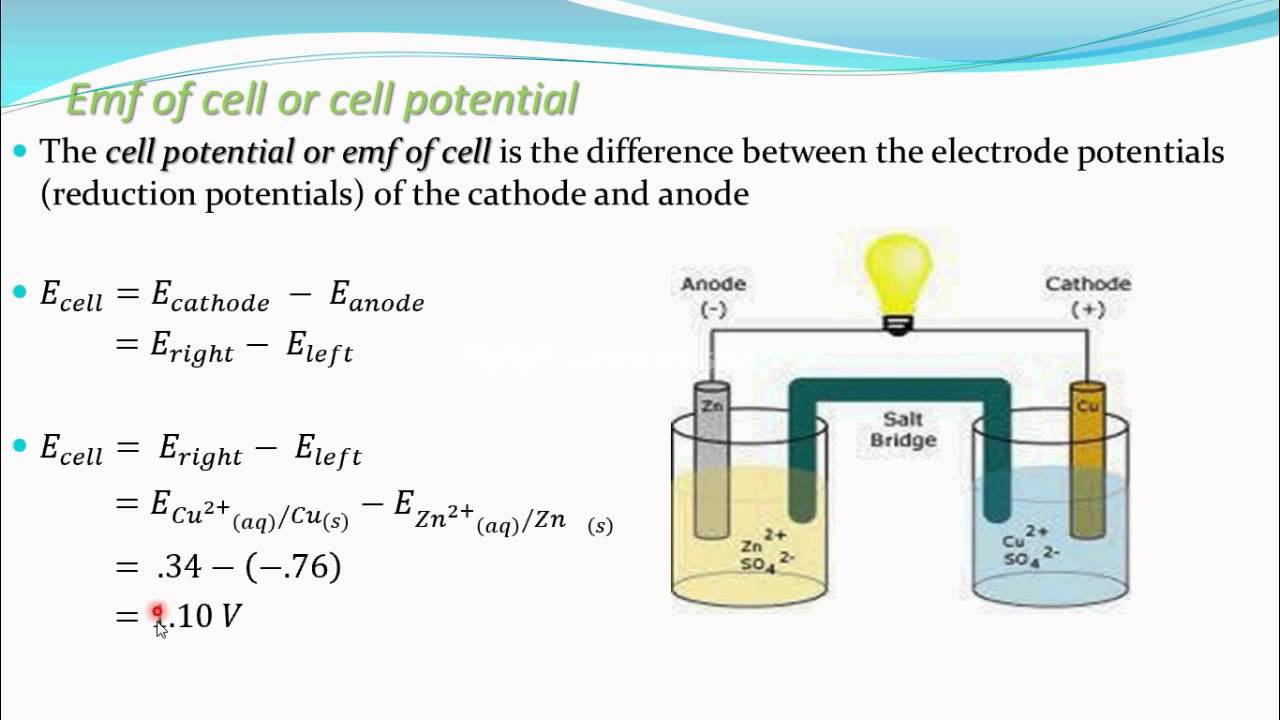
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12

How To Calculate E Cell | Sciencing

How to Calculate Cell Potential.
Calculate the E° cell for the following equation. Use these standard

Calculate E°cell for the following reaction at 298K: `2Al_((s)) + 3Cu

Emf of daniel cell from nernst equation(Electrochemistry part 28 for
